We use physics concepts to understand phenomena underlying important biological processes and functions, and to advance the use of innovative experimental technologies for their experimental investigation. Our focus themes at FIM include:
- Mechanics and Thermodynamics of Biological systems
Rheological properties of living cells
Impact of mechanical/thermodynamic properties on biological functions
Atomic Force Microscopy - Science using optical tweezers
Protein folding and refolding pathways
Functional dynamics of molecular chaperones
Faculty: Andrea Alessandrini, Ciro Cecconi, Elisabetta Salerno
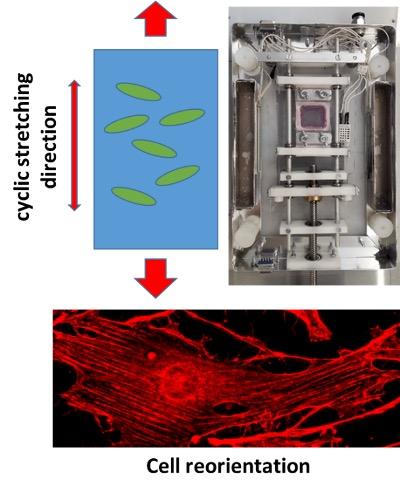
Mechanical forces are crucial for the behavior of biological systems. For example, cells continuously apply forces to the extracellular matrix and at the same time receive external mechanical stimuli. In our group we investigate rheological properties of living cells exploiting different biophysical techniques such as Micropipette Aspiration and Atomic Force Microscopy. We focus on the effect of potential drugs on the cytoskeletal organization of cells and on their migration properties. We also expose living cells to cyclic mechanical stretching and study the effect of periodic stimulation on the differentiation of stem cells that are physiologically subjected to a cyclic uniaxial or isotropic stretching.
The biological membrane separates the internal region of a cell from the external environment. Its mechanical and thermodynamic properties affect its biological functions. Many drugs and exogenous molecules in general, in addition to interacting with specific protein targets, can interact with the membrane and modify its mechanical or thermodynamic phase properties. In our group we use by Atomic Force Microscopy and Fluorescence Microscopy to study lipid bilayer model systems such as Supported Lipid Bilayers and Giant Unilamellar Vesicles and their interaction with potential anti-bacterial drugs such as antimicrobial- and lipo-peptides.
Image adapted from Journal of Colloid and Interface Science 553, 247 (2019).
People: Prof. A. Alessandrini
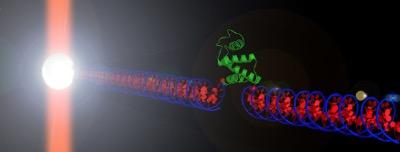
In the 30 years since the pioneering work of the Nobel laureate Arthur Ashkin, Optical Tweezers (OT) have evolved into a behemoth in the field of single molecule biophysics. Using a laser beam as both the probing as well as the spatial detection element, OT allows for the manipulation of single molecules with high spatial and temporal resolutions. At the Optical Tweezers Lab of UNIMORE, we employ OT to explore protein dynamics at single molecule level, with two major research threads:
- Study of protein folding and misfolding
Proteins are polypeptide chains that fold into functional three-dimensional structures through a molecular mechanism that remains poorly understood, despite the theoretical and experimental efforts of many scientists over the past 40 years. When the folding process goes wrong (misfolding) the protein becomes useless and often toxic for the organism. Using OT we study the folding and misfolding processes of different proteins under different experimental conditions, and characterize the energy landscapes underlying these reactions. - Investigation of the functional dynamics of molecular chaperones
Molecular chaperones are proteins that maintain cellular proteostasis by shielding client proteins against misfolding and aggregation. Some chaperones prevent protein aggregation (holdase action), others promote protein re-folding (foldase action). Using OT we characterize the molecular mechanisms underlying the holdase and foldase actions of different chaperones at the single molecule level, revealing useful information for the development of therapies against diseases caused by the malfunctioning of these proteins.
Image adapted from Advances in Protein Chemistry and Structural Biology 92, 93 (2013).
People: Prof C. Cecconi